Algae for Tilapia
Contents
- 1 Feeding
- 2 Algal growth
- 3 Selective feeding
- 4 Brachionus plicatilis
- 5 Keratella
- 6 Peridinium cinctum
- 7 Anomoeoneis sphaerophora
- 8 Diatoma vulgaris
- 9 Cyclotella
- 10 Melosira
- 11 Navicula
- 12 Nitzschia
- 13 Rhopalodia vermicularis
- 14 Anabaena
- 15 Spirulina
- 16 Microcystis
- 17 Periphyton
- 18 Algae meal
- 19 Off-flavor
Feeding

Tilapia start feeding shortly before dawn and feed continually (but most intensively between 12:00 and 18:00[7]) until about dusk. They do not feed during the night.[8][9] Tilapia (Oreochromis niloticus) may effectively control algal blooms in eutrophic waters.[10] Planktonic plants and animals make up the bulk of the diet under natural conditions.[11] Zooplankton (animal) does not exceed 1.5% of total stomach contents [12], with predation pressure being the greatest on Ceriodaphnia reticulata (small water fleas that feed on small algae) [13], due to low escape probabilities for (Cerio)daphnia compared to copepods (small crustaceans).[14] Phytoplankton (plant plankton) may contribute over 30% by volume of stomach contents of Oreochromis niloticus.[15] Small Tilapia rendalli consumed more diatoms than larger individuals.[16] Oreochromis niloticus feeds mainly on zooplankton until 5 cm long, and mainly on phytoplankton as it grows older. In small fish (<75 g.) Bacillariophyceae are the main phytoplankter, and in larger fish Chlorophyceae are the main phytoplankter.[17] Tilapia (Sarotherodon melanotheron) shift from visually feeding on zooplankton when juveniles to mostly filter feeding on phytoplankton when adults [18], consuming less Microcystis and Scenedesmus as they grow bigger.[19] Blue-green algae are common components of the Tilapia diet. In the stomach of Tilapia nilotica the cells of blue-green algae are lysed by high concentrations of acid (pH 1.4–1.9). After lysis, cell contents are digested in the intestine (by pepsinogen, a pancreatic α-amylase, trypsin, chymotrypsin and esterase activity). Acid is secreted in relation to feeding. Acid is not secreted by stressed fish.[20] Filtration rates of algae increases linearly as water temperature increases from 17°C to 32°C.[21]
Algal growth
The favourable pH range for plankton growth ranges between 6.5 and 7.5. Acidic conditions favor chlorophytes (green algae) while alkaline condition fosters nitrogen-fixing cyanobacteria.[22] High salinity favors green algae (plus Spirulina) while low salinity fosters cyanobacteria. In phytoplankton-based recirculating systems, algae may rather be limited by dissolved organic carbon availability rather than by nitrogen or phosphorus.[23] Chlorella vulgaris has higher lipid contents than Scenedesmus abundans and Monoraphidium minitum, which was also reflected in their predator, Brachionus calyciflorus. [24] Most fertilized fish ponds with dirty green water have more than 242 mg phytoplankton biomass per L water.[25] Diatoms are less dependent on trace elements than other algae.[26]
Selective feeding

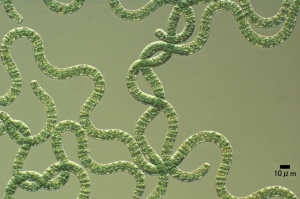
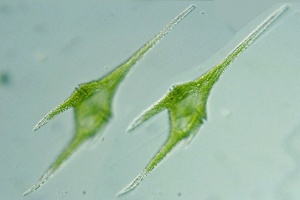
Tilapia feed selectively on large algae, mainly cyanobacteria and diatoms.[27] Oreochromis niloticus may select only Cyanophyta and Euglenophyta, with occasional Bacillariophyta.[28] Oreochromis niloticus may select Cyanophyta and sometimes select Bacillariophyta and Euglenophyta.[29] Blue tilapias (4.3 to 18.7 cm long) selectively feed on particles larger than 25 μm.[30] Nile tilapia is particularly effective in filtering the larger particle size taxa.[31] Larger phytoplankton (plant plankton) are being filtered proportionally more than the smaller phytoplankton, and cyanobacteria (in 100% of fish[32]) more than green algae (Scenedesmus, Ankistrodesmus, Tetraedron).[33] Tilapia aurea (Blue tilapia) favour Uroglenopsis sp. (cells forming hollow spherical colonies, > 400 μm diameter), Ceratium sp.(dinoflagellate (the least ammonium tolerant [34]), 310 μm long, 58 μm wide; optimum salinity 10 ppt[35]) and Keratella sp. over (small-sized algae) Rhodomonas sp., Chrysochromulina sp., Chlamydomonas sp., Cyclotella sp. and (zooplankter) Diaptomus sp.[36] Oreochromis niloticus juveniles prefer Elodea canadensis over Potamogeton pectinatus and Spirodela polyrhiza. Myriophyllum spicatum was the least preferred.[37]
Diatoms are microscopic unicellular algae, all consisting of the same basic parts; nucleus, cytoplasm, plasma membrane, and the cell wall.[38] Oreochromis niloticus prefer to prey on the large size diatoms, such as Melosira granulata, Syndra ulna (high Si and low P requirements[39]), Cyclotella ocellata and Cyclotella operculata.[40] Astrionella sp. and Rhopalodia vermicularis (bacillariophytes) and Peridinium sp. (dinophyte) were selected by both wild and pond-cultured Oreochromis niloticus. Lake fish particularly selected Astrionella sp., Aulacoseira (Melosira) nyassensis, Navicula sp. (which grow on sediment[41]) and Rhopalodia vermicularis (bacillariophytes). Pond fish particularly selected Ankistrodesmus falcatus (chlorophyte), Lyngbya circumcreta (cyanophyte) and Nitzschia acicularis.[42] Oreochromis niloticus may prefer Spirulina laxisma and Nitzschia accicularis the most. The least preferred food items may be Lyngbya circumcreta, Microcystis aeruginosa and Pediastrum simplex.[43]
The main species of algae found in Oreochromis niloticus stomach belonged to Cyanophyta (2nd most ammonium tolerant[44]), Chlorophyta (most ammonium tolerant[45]), Bacillariophyta and Euglenophyta.[46] The most frequent species represented in Oreochromis niloticus stomach are Anabaena sp., Merismopedia eleganse, Microcystis aeruginose, Nodularia harveyana and Oscillatoria sp. (Cyanophyta), Cerasterias sp., Chlorella spp., Crucigenia sp., Pediastrum spp., Scenedesmus spp. and Tetraedron sp. (Chlorophyta), Amphora ovalis, Cocconeis placentula, Cymatopleura solea, Cymbella cistula, Gyrosigma attenuatum, Melosira granulata, Navicula spp., Nitzschia spp., Pinnularia spp., Serurella sp. and Synedra sp. (Bacillariophyta) and Euglena and Phacus spp. (Euglenophyta).[47] The most frequent genera represented in fish (Oreochromis niloticus) stomach in all sizes were Anabaena, Anabaenopsis, Coleospharium, Merismopedia, Microcystis, Nodularia, Oscillatoria, Spirulina (Cyanobacteria), Actinostrum, Chlorella, Closterium, Coelastrum, Eudrina, Pandorina, Pediastrum, Scenedesmus, Shereoderia, Staurastrum (Chlorophyceae), Amphora, Cocconeis, Cymatopleura, Cymbella, Gyrosigma, Melosira, Navicula, Pinnularia, Synedra (Bacillariophyceae) and Euglena and Phacus (Euglenophyceae).[48] Botryococcus sp. (green algae)[49][50] and Melosira (a diatom; Bacillariophyceae)[51] may also be major food items in the diet of Oreochromis niloticus.
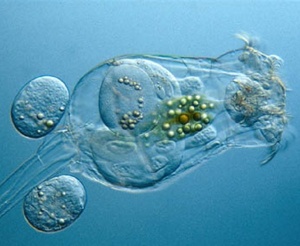
Brachionus plicatilis
Brachionus plicatilis is an important food source for larval fish. Brachionus plicatilis is also the most successful species of zooplankton used to control unicellular algal contaminants in Spirulina mass cultures even under conditions adverse to the growth of Spirulina (very low bicarbonate).[52] Brachionus plicatilis is a rotifer / zooplankton associated with saline, alkaline waters.[53] It may be 123 to 292 μm long and 114 to 199 μm wide. Compared to temperature and salinity, strain is the most important factor determining size.[54] Maximum reproduction occurs between 30 and 34 C. Reproduction rate is similar in 25% and 33% seawater. Brachionus plicatilis may thrive in 0.5 to 30 ppt salinity (brackish) and may be 99 to 281 μm long and may weigh 0.18 μg. In contrast to female Brachionus rubens, fecund female Brachionus plicatilis do not attach to a substrate. [55] Adult Brachionus plicatilis may be 213 to 315 μm long. Optimum salinity may be 18 ppt. The most common feed types are single-celled algae.[56] Brachionus plicatilis may (together with Nitzschia spp) survive in 10 pH, high calcium carbonate and 91 ppt salinity.[57]
Brachionus plicatilis may be mass cultured on Akashiwo sanguinea (Gymnodinium splendens / nelsoni), an unarmored (naked) dinoflagellate. [58] Akashiwo sanguinea is a mixotroph, as it is both photosynthetic and feeds on cyanobacteria and nanociliate populations (tiny protozoans)[59], which may be algivores.[60] It may form subsurface chlorophyll maximum layers in the water, which is consumed by larval fish.[61] This may be in response to a relative nitrate deficiency during the light period.[62] Dinoflagellates prefer calm water column situations; small-scale turbulence may diminish dinoflagellate growth and cell division, but may also increase cell size.[63] Akashiwo sanguinea may thrive in brackish conditions.[64] Coastal Akashiwo sanguinea bloom events may be supported by elevated urea.[65]
Keratella
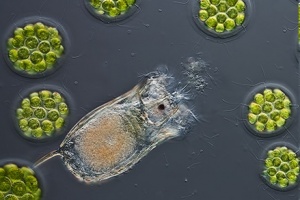
Keratella sp. are relatively small (~100 μm long) 'wheel animals' / rotifers / zooplankton. Growth may be optimal at 24°C temperature and 20 ppt salinity. Fecundity may be optimal at 0 ppt salinity.[66] Keratella hispida may thrive on 30 ppt. Keratella americana, Keratella tropica and Keratella cochlearis are considered euryhaline (able to adapt to a wide range of salinities).[67] Keratella quadrata and Keratella cochlearis are euryhaline and perennial (not seasonal).[68]
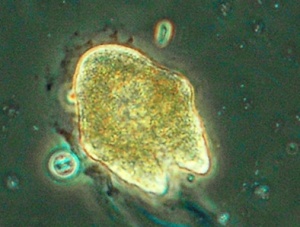
Peridinium cinctum
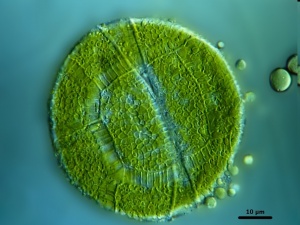
Peridinium species are large phytoplankton that less efficiently take up nutrients than smaller species. At their advantage is the capacity to modify their position in the water column (in accordance with light and nutrient availability), that they are (because of their large size) inedible by most zooplankton, and their extremely low phosphorus requirements (C:P molar ratio is 460:1).[69] They may produce 2 to 3 times more organic matter than other alga.[70]
Tilapia (galilaea) favourably consume Peridinium cinctum (30-70 μm armoured dinoflagellates).[71] Feeding rates of Tilapia galilaea and Tilapia aurea reach maximum values for zooplankton (animal plankton) and Peridinium cinctum.[72] Fingerlings fed experimentally on chlorophytes had lower growth rates compared with mosquito larvae, zooplankton and fresh Peridinium.[73] Growth rates of age-1 Tilapia galilaea on Peridinium may be 1–2% mean weight per day.[74]
When phosphorus is low, Peridinium has an advantage over other alga.[75] Peridinium aciculiferum may particularly produce toxins when deprived of phosphorus and in stationary phase.[76] Blooms of Peridinium gatunense (biomass > 100 g / m2) may change the color of the water to coffee brown in distinct patches and are associated with excessive oxygen super-saturation, and high phosphorus (> 0.05 mg/L) and nitrogen (> 1 mg/L).[77] Peridinium gatunense and Microcystis sp. are highly competitive.[78] Peridinium ponticum may thrive in 17 ppt salinity.[79] Peridinium cinctum is common below 20 ppt salinity.[80]
Anomoeoneis sphaerophora
Anomoeoneis sphaerophora (aka Navicula sphaerophora) may be 25-200 µm long and 12-60 µm wide.[81] Anomoeoneis sphaerophora var. rostrata may dominate in <50 cm shallow waters at 9.6 pH and 25.5-130 meq/L (carbonates + bicarbonates) alkalinity (908-4810 mg/L Na; 22-45 mg/L K; 7-8 mg/L Ca; 1-6 mg/L Mg; 0.4 mg/l Fe; 0.7-3 SiO2; 45-68 mg/L SO4; 674-2680 mg/L Cl).[82] Anomoeoneis sphaerophora var. sphaerophora is benthic and may dominate in 13 to 64 cm shallow fresh and brackish waters at pH 9.7 to 10.1 and 23.1ºC to 34.2ºC ; conductivity > 2870 µS/cm, and may also thrive in waters with lower conductivity (318-2110 µS/cm) and pH (7.0-8.3) [83]
Diatoma vulgaris
Diatoma vulgaris is 15-60 µm long and 8-12 µm wide and may develop in fresh and brackish water. It forms zig-zag colonies attached to the substrate by a mucilage pad. [84] Diatoma vulgaris is associated with Melosira species and may develop optimally in running water.[85]
Cyclotella
Oreochromis niloticus prefer to prey on the large size diatoms, such as Cyclotella ocellata and Cyclotella operculata.[86] Cyclotella operculata may thrive at 7.9 to 8.4 pH (Cl 64 mg/L ; NH3 0.28 mg/L ; P 0.23 mg/L).[87] Cyclotella meneghiniana is favoured by high light penetration [88] and most abundant at high nitrate (4.6 mg/L). (ph 7.7 to 8.0)[89] Cyclotella meneghiniana may thrive in shallow water (<50 cm) at 908-1100 mg/L Na; 22-33 mg/L K; 7-8 mg/L Ca; 4-6 mg/L Mg; 3-4 mg/L SiO2; 0.4 mg/L Fe; 45-48 SO4; 674-830 mg/L Cl and 25.5-26.5 meq/L alkalinity (carbonates plus bicarbonates).[90] Cyclotella pseudostellagera may thrive at low Cl (7 mg/L) and high P (59 mg/L, as PO4-), 023 mg/L nitrate, 0.26 mg/L NH3.[91]
Melosira

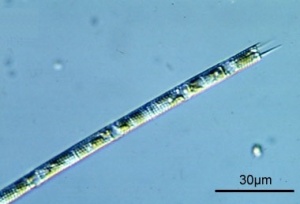
Oreochromis niloticus prefer to prey on the large size diatoms, such as Melosira granulata (Aulacoseira granulata).[92] One single cell may be 22 to 37 µm long and 3.5 to 5 µm in diameter.[93] Melosira granulata is often found in turbid water.[94] Melosira sp. are meroplanktonic, meaning that they are part of plankton only during the larval stage of their life and spend their adult lives in between the plankton on the reef, which they may enter due to wind-induced mixing.[95] Melosira have high-nutrient requirements and may occur in completely mixed shallow eutrophic waters. Melosira sp in general are characteristic for low salinity and low alkalinity waters.[96] Melosira granulata may thrive at 7 pH, 110 mg/L bicarbonate alkalinity and 260 mg/L hardness [97] / at 7 to 8 pH [98] / 7.2 to 8.5 pH (total bicarbonate alkalinity: 80 to 170 mg/L) [99] / 7.9 to 8.4 pH (Cl 64 mg/L ; NH3 0.28 mg/L ; P 0.23 mg/L) [100] / at 8 to 9 pH.[101] Melosira granulata is associated with freshwater lakes [102], but Melosira granulata may also be considered euryhaline (may thrive in a wide range of salinities).[103][104] Melosira granulata is more abundant at higher temperatures. [105] Melosira granulata is less seasonal than offshore populations of Melosira victoriae and Melosira nyassensis.[106]
The principal limiting resources of Melosira are light and phosphorus (and silica).[107] Melosira distans and Melosira ambigua need much light and have low phosphorus requirements and Melosira nyassensis needs the least light and the most phosphorus. Melosira granulata (which may adjust to light availability) and Melosira agassizii are intermediate.[108] Melosira italica v. valida develops optimally in running water (reophilous).[109]
Navicula radiosa is about 70 µm long and 10 µm wide. It thrives at 21 to 27°C. It is often found in littoral waters and is indifferent of pH (>7 pH) and cationity.[110] Navicula cryptocephala is favoured by high light penetration [111] and is considered eurytopic (the ability to exist in a wide range of chemical environments) and may thrive at 0.15 to 4.6 mg/L nitrate, 7 to 65 mg/L chloride, 0.13 to 0.59 mg/L total phosphorus (as PO4-) and 0.00 to 0.28 mg/L NH3 [112], and in shallow water (<50 cm) at 1100 mg/L Na; 33 mg/L K; 8 mg/L Ca; 4 mg/L Mg; 3 mg/L SiO2; 45 SO4; 830 mg/L Cl and 26.5 meq/L alkalinity (carbonates plus bicarbonates).[113] Navicula placentula may occur in shallow waters.[114]
Nitzschia

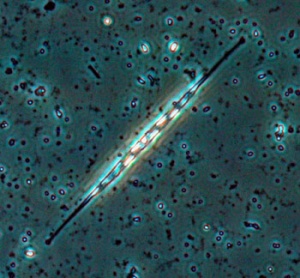
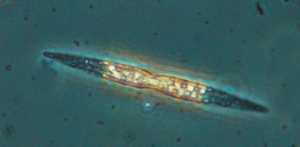
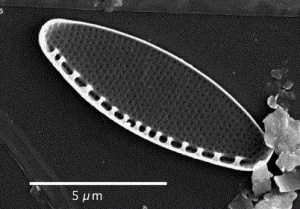
Oreochromis niloticus may prefer Spirulina and Nitzschia accicularis the most.[115] Nitzschia accicularis is a diatom (100 to 500 µm long), belonging to the Bacillariophyta (phytoplankton).[116] Nitzschia are Pennatophycidae, which are epipelic diatoms; benthic (=not free floating) diatoms growing on sediment.[117] Upper ammonium tolerance of diatoms may be 3600 μM.[118] Nitzschia are intermediates along the silicon:phosphorus gradient; with relatively balanced silicon and phosphorus requirements.[119] Unlike most diatoms, Nitzschia fonticola is a nitrogen heterotroph (requiring organic nitrogen).[120]
Nitzschia accicularis may tolerate high salinity.[121] Optimum photosynthetic activity by Nitzschia closterium is at 10 ppt [122], but it may tolerate salinity over 35‰ (ppt).[123] Optimum salinity for Nitzschia americana growth is 26 ppt.[124] Nitzschia ovalis may grow at salinities from 5–40‰ (ppt).[125] Nitzschia fonticola is euryhaline and may thrive in 7 to 50 ppt.[126]
Nitzschia species are not dominant at temperatures below 23°C.[127] Many Nitzschia species stop growing at pH values of 8.7 to 9.1, and data suggest that smaller species have a higher upper pH limit for growth than larger species.[128] Most Nitzschia may survive in 10 pH.[129] Particularly Nitzschia dissipata and Nitzschia denticulata (and Nitzschia accicularis to a lesser extend) may thrive at 7.2 to 8.5 pH (total bicarbonate alkalinity: 80 to 170 mg/L).[130] Nitzschia archibaldii may thrive at 7.1 to 8.2 pH, 0 to 15 mg/L carbonates plus 110 to 170 mg/L bicarbonates alkalinity and 188 to 260 mg/L hardness.[131] Nitzschia bolivianais is epilithic; growing on rocks.[132] Nitzschia sigma is associated with saline, alkaline waters.[133] Nltzschia linearis (related to Nitzschia palea) is truly rheobiontic (especially found in running water).[134]
Nitzschia frustulum develops optimally in stagnant waters (limnophilous).[135] Nitzschia frustulum (aka Synedra minutissima / perpusilla / quadrangula) is both benthonic and associated with Spirulina platensis blooms. Nitzschia frustulum is a nitrogen heterotroph and dense populations of Spirulina platensis release organic nitrogen. In the presence of Spirulina platensis (so that the diatom can enter the plankton), Nitzschia frustulum is the dominant diatom in saline lakes with alkalinity 80 > mEq/L, but also survives in freshwater.[136]
Nitzschia sp. 1 concentrations occur when input water has high Si:P ratios (warm, wet climate).[137] Nitzschia sp. 1 (only 85 µm long) and Nitzschia hungarica (aka Tryblionella hungarica; 35-130 µm long [138]) are benthonic and only dominant in extremely shallow (saline, alkaline) waters.[139]
Nltzschia palea may be the most resistant and most tolerant of all diatoms. It is eurytopic (indifferent of chemical conditions), euryhaline (indifferent of salinity) and eurythermlc (indifferent of temperature) and grows in polluted water (but containing moderate dissolved oxygen) forming a brown surface layer on rocks of rapids, as well as in quiet water on shallow silt banks.[140] Nitzschia palea may occur in shallow waters [141] and domination is reed-associated. Nitzschia palea may dominate in <50 cm shallow waters at 9.6 pH and 25.5-130 meq/L (carbonates + bicarbonates) alkalinity (908-4810 mg/L Na; 22-45 mg/L K; 7-8 mg/L Ca; 1-6 mg/L Mg; 0.4 mg/l Fe; 0.7-3 SiO2; 45-68 mg/L SO4; 674-2680 mg/L Cl).[142] Nitzschia palea (19-53 µm long, 3.8-5 µm wide) may dominate in 13 to 44 cm shallow fresh water at pH 7.0 to 8.5, and at 23 to 32°C (Optimum range: 22.8-29.5°C); conductivity 430- 2110 µS/cm.[143] Nitzschia palea has its maximum rate of photosynthesis at 33°C and is strongly inhibited at 40°C.[144] Nitzschia palea is considered eurytopic (the ability to exist in a wide range of chemical environments) and may thrive at 0.15 to 4.6 mg/L nitrate, 7 to 65 mg/L chloride, 0.13 to 0.59 mg/L total phosphorus (as PO4-) and 0.00 to 0.28 mg/L NH3. [145]
Some (Pseudo)Nitzschia species produce domoic acid, a neurotoxin that may cause amnesic shellfish poisoning in humans. Nitzschia pungens [146], Nitzschia pseudodelicatissima [147]) and particularly Pseudo-nitzschia species in high pH conditions [148], such as the cosmopolites Pseudo-nitzschiaseriata delicatissima and Pseudo-nitzschiaseriata pseudodelicatissima, but most notably the cosmopolites Pseudo-nitzschiaseriata australis and Pseudo-nitzschiaseriata multiseries [149] may produce domoic acid in the presence of light.[150] Pseudo-Nitzschia may tolerate up to 45 ppt salinity.[151]
Rhopalodia vermicularis
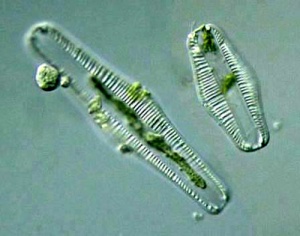
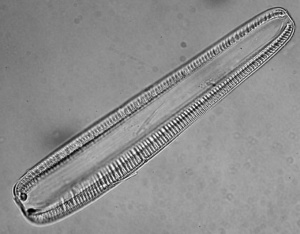
Other diatoms that thrive in (moderately saline and moderately alkaline) shallow water are Rhopalodia vermicularis and Cocconeis placentula. [152]. Rhopalodia vermicularis and Rhopalodia gracilis may dominate in shallow waters.[153] Rhopalodia spp. are littoral diatoms, thriving in brackish water.[154] Rhopalodia vermicularis grows in fresh to hyperalkaline lakes; 7 to 9.5 pH and 10 to 100 meq/L alkalinity (sodium bicarbonate or calcium- plus magnesium bicarbonate), at 21 to 27°C. [155] Rhopalodia gibba may grow at pH 10 and low alkalinity; 9.[156] Despite their low abundance in pond water, both wild and pond-cultured Oreochromis niloticus preferentially selected Rhopalodia vermicularis.[157] Rhopalodia is endemic to East Africa [158] and may be 80-150 μm long.[159] Rhopalodia sp are epipelic algae; growing on sediment.[160]
Anabaena

Anabaena sp. are large filamentous and neurotoxin-producing cyanobacteria (blue-green algae) that exist as plankton. Feeding mostly on Anabaena spiroides, Oreochromis niloticus (mean total weight: 31.5 g) may ingest 5.1% body mass equivalent (wet weight basis).[161] Ingestion rates are higher on Anabaena cylindrica than on Microcystis aeruginosa.[162] Anabaena flos-aquae is much better assimilated (83%) than green algae. Gross growth efficiency of fish fed Anabaena flos-aquae was 46% in comparison to fish fed Chlamydomonas (22%) and Ankistrodesmus falcatus; 24%.[163] Tilapia grow much better on Anabaena than on Clamydomonas (green algae) [164] Nile Tilapia fed ration equal 3% of the total fish body weight, containing 1.5% Anabaena had higher growth and were relatively protected against A. hyrophila infection.[165] Anabaena circinalis may cause off-flavor in fish flesh.[166] In competition experiments between Anabaeana and toxic Microcystis aeruginosa, dominancy is mainly determined by the initial biomass ratio.[167] Anabaena circinalis and Anabaena spiroides thrive in salinity less than 3 ppt.[168] Anabaena tortulosa is relatively tolerant of salinity up to 15 ppt.[169] Anabaena spp. (and Anabaenopsis) are common in alkaline saline lakes. [170] Salt tolerance in cyanobacteria is increased by nitrogenous compounds (inhibiting Na+ influx), but by nitrate more so than by ammonium or glutamine.[171]
Spirulina

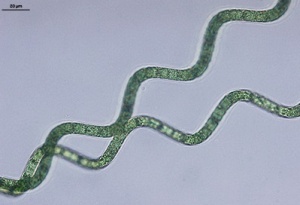


Spirulina (Arthrospira) are blue-green algae/cyanobacteria and a rich source of protein and potassium.[172] Spirulina platensis contains 50 to 65% protein (10% is non-protein nitrogen)[173], is high in vitamin B1 and B6 and is 85 to 95% assimilated. It may contain up to 14% lipids (of dry mass). [174] Spirulina platensis growth is optimum below 10.5 pH.[175] Spirulina platensis is naturally found in tropical regions inhabiting alkaline lakes (pH 9.4 to 11 ; HCO3 + CO2 > 51 meq/L [176]) with high concentration of NaCl and bicarbonates [177], but may adapt to very different habitats, including freshwater.[178] In alkaline conditions, at higher salt levels (2.5 to 30 g. salt / L; mostly carbonates and bicarbonates), Spirulina becomes more dominant (with Anabaenopsis, Oscillatoria, Synechocystis), or even the only significant organism (> 30 g. salt / L) Optimum salinity may be between 20 and 70 ppt. [179] Oreochromis niloticus may fairly tolerate salinity up to 10 ppt [180] Tilapia may tolerate 0.5% salinity (5 ppt).[181] At 8 g / L salinity adult Oreochromis niloticus may be stocked at 40 fish / m3. At 15 g/L salinity total production is more than halved.[182]
Spirulina platensis (small biomass) may be grown on human urine.[183] At pH 8.4, urea may be used as a source of nitrogen up to 1.5 g./L [184], the best growth temperature being 29°C [185] at an appropriate slowly increasing urea feeding rate.[186] Regarding phosphorus, maximum biomass production at high light intensity was observed at 250 mg/L K2HPO4.[187] The optimum nitrogen:phosphorus ratio is 7:1 at optimum light intensity.[188] The temperature range for optimum Spirulina growth is 30 to 35°C.[189] At 35°C there is a negative effect on biomass production but a positive effect on the production of protein, lipids and phenolics.[190] Spirulina may be cultivated in water with 16 g/L baking soda, 2 g/L Potassium nitrate, 1 g/L sea salt, 0.1 g/L potassium phosphate and 0.01 g/L iron sulphate. [191] In an open raceway system dissolved oxygen may reach 30 mg/L at 28°C. Above 25 mg/L Spirulina growth is inhibited.[192] During a period of nitrogen starvation, Spirulina platensis may survive and grow unaffected based on/due to prior storage of nitrogen in pigment proteins (C-Phycocyanin).[193]
Larval tilapia that were fed solely raw Spirulina at a feeding rate of 30% (on a dry basis) of bodyweight in the first 3 weeks, 10% in weeks 4–6, and 3% in weeks 7–10, kept growing without any abnormality[194], at a slightly lower rate than fish fed fishmeal. Fish fed Spirulina had higher protein, polar lipid, linoleic acid and γ-linolenic acid contents and lower ash and omega-3 contents than fish fed the commercial diet.[195] Elastic modulus of flesh of the Spirulina-fed fish was significantly higher and viscosity lower.[196] Larval Tilapia (Oreochromis niloticus < 3.4 cm) prefer consuming Spirulina platensis over Euglena gracilis (flagellate protists), and is also more readily assimilated. Tilapia prefer both species over Chlorella vulgaris (green algae, 45% dry matter protein), which is hardly ingested by larval tilapia.[197] Tilapia prefer Spirulina over Navicula (a diatom).[198] In Tilapia aurea fed Spirulina platensis, food conversion was 2.0.[199] Dietary Spirulina incorporation increases antioxidant activity in tilapia.[200] When live Spirulina partly replaces commercial feed, fish mortality due to Aeromonas hydrophila infection decreases with an increase in the Spirulina level in the diet.[201]
Microcystis
In lakes where Microcystis spp. are the most abundant, Microcystis sp. may account for more than 80% of ingested phytoplankton.[202] Various cyanobacteria may produce toxins (eg Microcystis aeruginosa). Average digestion rate of Microcystis aeruginosa (cyanobacteria producing neurotoxins and hepatotoxins) by Tilapia fingerlings is 58 to 78% at 25℃.[203] In Oreochromis niloticus a high rate of defaecation of undigested Microcystis may occur.[204]
Microcystis aeruginosa may survive in a wide range of salinities.[205] Oreochromis mossambicus exposed to extracts from Microcystis aeruginosa showed significant plasma hypocalcaemia.[206] Toxin producing cyanobacteria do not (negatively) affect growth, feed conversion efficiency, health or mortality in Oreochromis niloticus. Microcystins do accumulate in muscle tissue (and particularly in the liver), which may exceed the upper limit of the tolerable daily intake of microcystins suggested by the WHO (0.04 μg/kg body weight/d).[207] Accumulation of microcystis increases lipoxidation (a biomarker of oxygen-mediated toxicity) in the liver.[208] Microcystin concentrations in the liver and intestine may gradually decrease as a result of the capacity in Tilapia to depurate and excrete mycrocystins into the bile and surrounding water, to avoid toxicity.[209] During the depuration period, muscle tissue may contain the highest concentration of microcystins, which may exceed the tolerable limit for humans (even in combined diets).[210] Microcystis wesenbergii is non-toxic, less competitive, but more suited to low phosphorus environments than Microcystis aeruginosa.[211] Microcystis flos-aquae is more dominant in salinity above 3 ppt, whereas Microcystis_aeruginosa is dominant in salinity up to 3 ppt.[212]
Periphyton
Periphyton is a mixture of algae, cyanobacteria, heterotrophic microbes and detritus (mainly scraps of macrophytes and mud [213]) attached to submerged surfaces embedded in a slimy mucopolysaccharide matrix.[214] Oreochromis niloticus graze actively, with little selectivity, on the periphyton established on artificial substrates in cages. Both animal and plant material are removed to an equal extent, but less smaller particles are ingested.[215] Surface-grazing on periphyton is greater than on Microcystis aeruginosa. For tilapia, filter-feeding may be a relatively unimportant method of ingesting algae.[216] Tilapia mossambica feeds almost exclusively on periphytic detrital aggregate. (Assimilation efficiencies: organic matter 63%, protein 77%, carbohydrate 63%) [217] Feed conversion ratio of dry matter periphyton may be 2.81. Fish growth may be sustained on periphyton despite 55% (dry matter) ash content.[218] The fresh microbial mat was 81% digestible by Nile tilapia, comparing favorably with commercial catfish feed in digestibility by Nile tilapia. The dried form was significantly less digestible.[219] Tilapia are equiped with a 'stomach bypass' to be able to bypass or regurgitate unwanted materials.[220]
Algae meal
Under most unnatural feeding conditions tilapia are unable to sufficiently ingest high volumes of algae. They may need constant grazing to fulfill their nutrient requirements.[221] Tilapia (Sarotherodon niloticus) grow better on fishmeal (fish meal may contain 74% protein and 8% lipids.[222]) than on a 25% protein green algae meal (Cladophora glomerata). Weight gain decreased as the level of algal protein increased as replacement of fish meal. Protein digestibility was highest on a 5:1 ratio (fishmeal : green algae meal).[223] Protein synthesis (with normal sulfur and carbon content) by green algae during the night may match protein synthesis during the day (in Dunaliella tertiolecta).[224] Protein derived from algae does not promote adequate growth in Rainbow trout.[225] Fish fed 5% ulva meal (Green algae; Ulva rigida) showed increased growth, feed conversion ratio and protein efficiency ratio.[226] Ulva meal may replace soy bean meal to the extend of 20% without negatively affecting growth of male larval tilapia. Feed conversion ratio increased with increasing ulva meal content.[227] Green algae meal (Hydrodictyon reticulatum) may replace meal to the extend of 25% without negatively affecting growth of Oreochromis niloticus and Tilapia zillii fingerlings.[228] Spirulina maxima meal protein can replace up to 40% of the fish meal protein in Oreochromis mossambicus fry diets without negatively affecting growth.[229]
Off-flavor
Cyanobacteria causing off-flavor (by geosmin and 2-methylisoborneol, MIB in fish flesh) are Oscillatoria tenuis, Anabaena circinalis and Pseudanabaena catenata. Tilapia flesh derived from cage culture provided a lower possibility of off-flavor contamination than those from earthen ponds.[230]